What is Immunoprecipitation (IP)?
Immunoprecipitation (IP) is a specialized laboratory technique that holds a pivotal role in modern biological and biomedical research. This method allows researchers to selectively extract and study specific proteins or protein complexes from complex biological samples, providing invaluable insights into cellular processes, protein interactions, and disease mechanisms.
At its core, immunoprecipitation is a technique that harnesses the specificity of antibodies to isolate and concentrate proteins of interest from a heterogeneous mixture. This is achieved by forming antibody-antigen complexes and subsequently precipitating these complexes out of the sample. The proteins within these complexes can then be analyzed to glean vital information about their structure, function, and interactions.

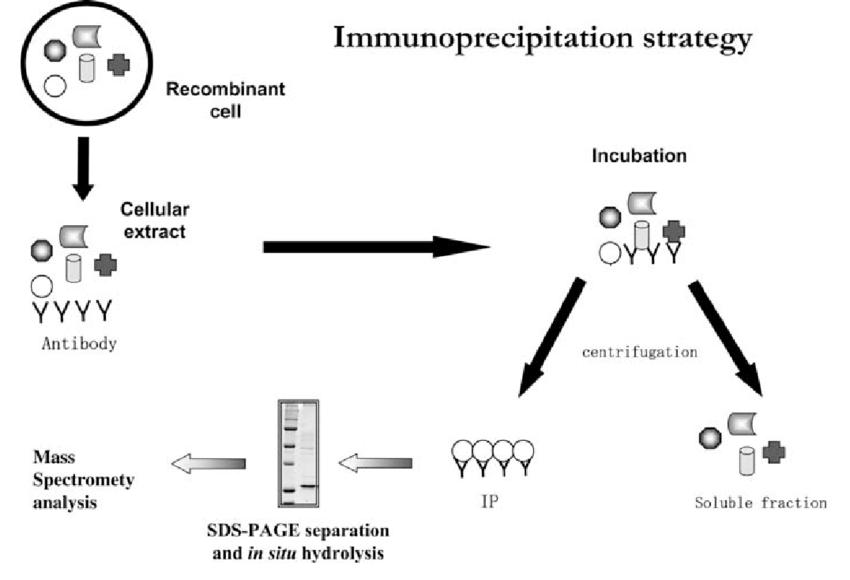
Outline of the immunoprecipitation strategy (Monti et al., 2005).
The Basic Principles of Immunoprecipitation
Immunoprecipitation (IP) is a multifaceted molecular biology technique rooted in the selective capture and isolation of specific proteins or protein complexes from a complex mixture of biological molecules. This intricate methodology hinges on the remarkable specificity of antibodies for their cognate antigens and forms the bedrock of numerous applications in the fields of molecular biology, biochemistry, and proteomics.
1. Antigen-Antibody Interaction
The crux of immunoprecipitation lies in the specific binding of an antibody to its target antigen. This interaction is driven by the antigenic determinants, epitopes, present on the target protein’s surface. Antibodies, which can be polyclonal or monoclonal, are carefully selected based on their ability to recognize and bind to the antigen of interest. The specificity of this interaction is the linchpin of the entire process, as it ensures that only the intended target is isolated during immunoprecipitation.
2. Formation of Antigen-Antibody Complexes
To initiate the immunoprecipitation procedure, the selected antibody is introduced into the biological sample, which typically contains a multitude of proteins. In the presence of the antibody, the specific antigen of interest forms an antigen-antibody complex. This complexation event is the result of molecular recognition and lock-and-key fit between the antibody and its target protein, akin to a precise puzzle piece connection.
3. Precipitation Agents
Once the antigen-antibody complexes have been formed within the sample, the next crucial step involves their selective separation from the surrounding milieu. To achieve this, precipitation agents are introduced. These agents, commonly in the form of protein A/G-coated agarose beads or magnetic beads, have an inherent affinity for the constant region of antibodies (Fc region). Consequently, they readily bind to the antibodies in the antigen-antibody complexes.
4. Washing to Remove Non-Specific Interactions
To ensure the specificity and purity of the isolated complexes, multiple washing steps follow the introduction of precipitation agents. These washes serve the critical purpose of eliminating any non-specific interactions or contaminants that may have adhered to the complexes during the process. Only the complexes where a bona fide antigen-antibody interaction exists remain bound to the precipitation agents.
5. Elution of Target Proteins
The final step involves the elution of the target proteins from the isolated antigen-antibody complexes. This is typically achieved by altering the conditions of the system, such as adjusting pH or introducing competitive agents. The eluted proteins, now separated from the precipitation agents, are collected and available for further analysis, whether it be for protein identification, quantification, or downstream experiments.
The Crucial Role of Antibodies in Immunoprecipitation
Immunoprecipitation (IP) heavily relies on the use of antibodies, which play a pivotal role in the specificity and success of the technique. Antibodies are critical components throughout the IP process.
Antibody Specificity: Antibodies possess an exceptional ability to recognize and bind to a specific antigen or target protein with high specificity. This specificity is derived from the variable regions of the antibody, enabling the discrimination of the target from other proteins in the sample.
Antibody Selection and Validation: Selecting the right antibody is paramount. Researchers must consider the antibody’s affinity, specificity, and suitability for the experimental system. Monoclonal and polyclonal antibodies offer different advantages, with monoclonal antibodies being highly specific to a single epitope and polyclonal antibodies recognizing multiple epitopes on the target protein.
Antibody-Antigen Complex Formation: In IP, the primary antibody forms a complex with the target protein. This complex is the basis for isolating the target protein from the sample. The antibody’s binding affinity ensures the specific capture of the protein of interest.
Antibody Isotype and Species: The choice of antibody isotype and species matters. Different IP techniques may require specific isotypes, and the antibody’s species should match the source of the sample. For example, protein A/G beads are often used to capture antibodies of different species.
Antibody Validation: To ensure that the antibody recognizes the target protein accurately, validation is essential. This involves confirming that the antibody is suitable for IP through techniques like Western blotting, immunofluorescence, or other assays.
Precipitation Agents and Their Selection
Precipitation agents are vital components in immunoprecipitation, as they enable the isolation of antigen-antibody complexes. The choice of the right precipitation agent is critical for the success of the IP experiment.
Characteristics of Precipitation Agents:
Protein A/G Beads: These beads are commonly used in immunoprecipitation due to their affinity for the Fc region of antibodies. They are versatile and suitable for a wide range of antibody isotypes and species.
Magnetic Beads: Magnetic beads coated with protein A, protein G, or specific antibodies are gaining popularity. They offer the advantage of easy handling and can be separated using a magnetic rack.
Selecting the Right Precipitation Agent:
Antibody Isotype and Species: The choice of precipitation agent should match the antibody’s isotype and species. Different precipitation agents have varying affinities for antibodies, so selection should align with the antibody used.
Experimental Goals: The nature of the experiment influences the choice of precipitation agent. For example, if the goal is to isolate antibodies, protein A/G beads are appropriate. If isolating a specific protein from the sample is the aim, magnetic beads with protein-specific antibodies may be more suitable.
Sample Complexity: The complexity of the sample and the potential for non-specific interactions may guide the choice of precipitation agent. Magnetic beads, with their higher specificity, may be advantageous for complex samples.
Different Types of Immunoprecipitation Techniques
| Technique | Principle | Applications |
| Co-Immunoprecipitation (Co-IP) | Isolation of protein complexes | – Study protein-protein interactions – Uncover cellular pathways and regulatory networks |
| Chromatin Immunoprecipitation (ChIP) | Isolation of DNA/RNA bound by proteins | – Epigenetic research – Gene regulation studies – Transcriptional control mechanisms |
| RNA Immunoprecipitation (RIP) | Study RNA-protein interactions | – Post-transcriptional gene regulation – Analysis of RNA-binding protein roles in RNA metabolism |
| Phospho-Immunoprecipitation (Phospho-IP) | Isolation of phosphorylated proteins | – Investigation of signaling pathways and kinase-substrate interactions – Study phosphoproteins and their roles |
| Cross-Linking Immunoprecipitation (CLIP) | Study RNA-protein interactions | – Understanding RNA metabolism and RNA-binding protein binding sites on RNA molecules |
| Tandem Affinity Purification (TAP) | Enhanced specificity and purity | – Complex protein-protein interaction studies – Protein identification and isolation of multi-subunit complexes |
| Immunoprecipitation-Mass Spectrometry (IP-MS) | Isolation of proteins followed by mass spectrometry | – Comprehensive protein identification and quantification – Proteomic studies of complex biological samples |
Applications of Immunoprecipitation in Biology
Protein Interaction Studies: IP is essential for probing protein-protein interactions. For instance, by immunoprecipitating a protein of interest (e.g., an oncogene) from a cell lysate, followed by mass spectrometry, researchers can identify interacting partners, elucidating pathways implicated in diseases like cancer.
Epigenetics Research: In epigenetics, Chromatin Immunoprecipitation (ChIP) is used to analyze DNA-protein interactions. For example, ChIP followed by quantitative PCR (ChIP-qPCR) can reveal the binding of histone modifications to specific gene promoters, shedding light on gene expression regulation.
RNA Research: RNA Immunoprecipitation (RIP) is crucial for studying RNA-protein interactions. By RIP-sequencing a RNA-binding protein (e.g., an RNA splicing factor), researchers can identify the RNA targets and understand post-transcriptional gene regulation.
Post-Translational Modifications (PTMs): IP can be tailored to decipher protein PTMs. Immunoprecipitating a phosphorylated protein, followed by Western blot analysis with a phospho-specific antibody, helps elucidate signaling pathways, such as the activation of kinases in cell signaling.
Disease Mechanisms: IP aids in the identification of disease biomarkers. For instance, in Alzheimer’s disease research, IP has been used to isolate and identify amyloid-beta protein, a key component in the formation of brain plaques associated with the disease.
Drug Development: In drug development, IP is instrumental in target identification. By immunoprecipitating a protein thought to be involved in a particular disease pathway, researchers can assess the feasibility of targeting this protein for drug development.
Biological Insights: IP deepens our understanding of biological systems. For example, in elucidating the functions of centrosomal proteins in cell division, IP can reveal protein-protein interactions critical for the organization of the mitotic spindle.
Reference
- Monti, Maria, et al. “Interaction proteomics.” Bioscience reports 25 (2005): 45-56.
Read Other: